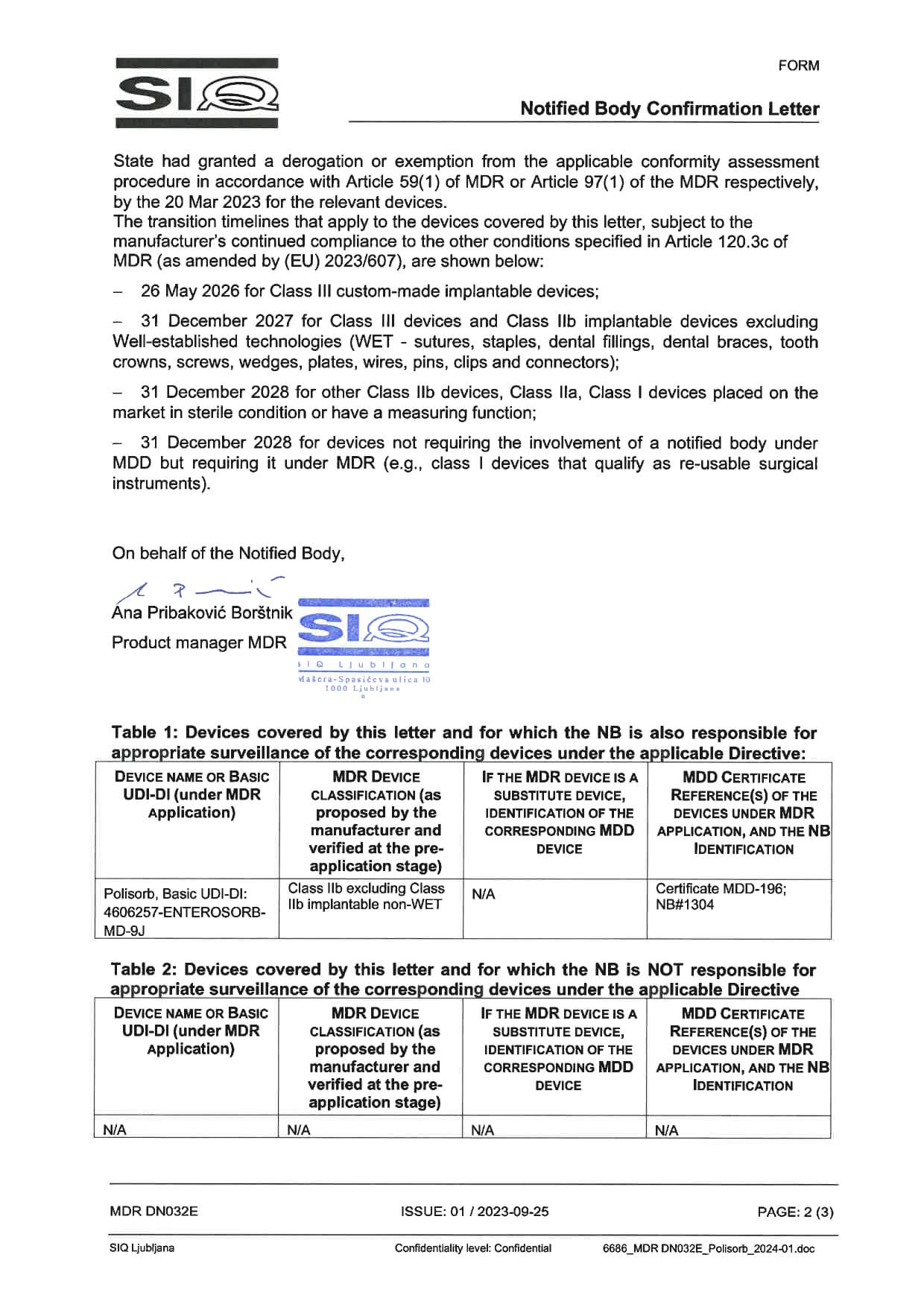
Certificates “Polisorb”
Polisorb complies with all medical requirements and standards for medicinal products. Compliance is confirmed by valid EU certificates in quality assurance of production and distribution of entersorbents.

Patent
Polisorb is a patented substance based on colloidal silicon dioxide
Research
Polisorb has undergone more than 50 clinical trials for efficacy and safety
Scientific works
There are more than 200 research works on Polisorb in the scientific field
No prescription
Polisorb is no prescriptive drug with no age restrictions
Action
Polisorb combines harmful bacteria, allergens, toxins and removes them from the body
Features
Polisorb has the best sorption and detoxification features among sorbents
EU Certificate - Quality Assurance
The certificate determines the compliance of medical products with the sphere of their use and establishes the basic requirements. This document authorizes the manufacturer of a medicinal product to place legally and freely and move its products within the European Union.
SIQ conducted a quality system audit in accordance with App V of the MDD and found that the manufacturer Polisorb meets the requirements of Directive 93/42/EEC.
Joint Stock Company “POLISORB”
ISO 13485:2016 - quality management system is an international standard regulating the activities of organizations involved in the development, production, packaging, storage, transport and disposal of medical devices.
SQ Ljubljana issued a certificate to IQNet that “POLISORB” has implemented and maintains a quality system for medical products in the field of production and distribution of enterosorbents (non active ingestion medicine).